Ideje Atom Vs Ion Diagram Zdarma
Ideje Atom Vs Ion Diagram Zdarma. An atom can be an ion, but not all ions are atoms. Neutral atoms can be turned into positively charged ions by. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.
Prezentováno Atom Vs Ion Hydrogen Carbonate Ion Structure Hd Png Download Kindpng
Atom is the basic unit of matter. The protons and neutrons form the nucleus of the atom while … Neutral atoms can be turned into positively charged ions by. Ions would therefore be either positive or negatively charged.But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.
Ions are atoms where the protons and the electrons are not equal. An atom can be an ion, but not all ions are atoms. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. But before that one needs to be clear of what is an atom and ion. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.

When sodium atoms form ions, they always form a 1.. Ions would therefore be either positive or negatively charged. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. When an ion is formed, the number of protons does not change. When sodium atoms form ions, they always form a 1. Atom is the basic unit of matter.

Neutral atoms can be turned into positively charged ions by. A group of atoms makes up matter. An atom is composed of protons, neutrons and electrons. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Molecules are groups of two or more atoms that are chemically bonded. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.

When an ion is formed, the number of protons does not change... When an ion is formed, the number of protons does not change. This nucleus carries the entire mass of the atom.
Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Molecules are groups of two or more atoms that are chemically bonded. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. According to his atom diagram, the atom has a small, positively charged nucleus in center. Ions would therefore be either positive or negatively charged. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.

But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. When sodium atoms form ions, they always form a 1. Neutral atoms can be turned into positively charged ions by.

According to his atom diagram, the atom has a small, positively charged nucleus in center... In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. When an ion is formed, the number of protons does not change.

Neutral atoms can be turned into positively charged ions by. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. This nucleus is surrounded by electrons revolving in orbits. According to his atom diagram, the atom has a small, positively charged nucleus in center. An atom can be an ion, but not all ions are atoms... But before that one needs to be clear of what is an atom and ion.

Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Atom is the basic unit of matter. Neutral atoms can be turned into positively charged ions by. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. When an ion is formed, the number of protons does not change.

Atom is the basic unit of matter. A group of atoms makes up matter. This nucleus carries the entire mass of the atom.

In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have... Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a... Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.

A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus... The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.

The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. But before that one needs to be clear of what is an atom and ion. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. Ions would therefore be either positive or negatively charged. Neutral atoms can be turned into positively charged ions by. Second, most atoms form ions of a single characteristic charge. These atoms have a nucleus, with protons and neutrons in it.. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.
The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. The protons and neutrons form the nucleus of the atom while … Second, most atoms form ions of a single characteristic charge.

Atom is the basic unit of matter. . Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus.

When sodium atoms form ions, they always form a 1... A group of atoms makes up matter.. A group of atoms makes up matter.

Molecules are groups of two or more atoms that are chemically bonded. Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. When sodium atoms form ions, they always form a 1. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. Ions are atoms where the protons and the electrons are not equal. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Molecules are groups of two or more atoms that are chemically bonded.

Atom is the basic unit of matter. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.
Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. This conclusion helped him propose 'rutherford's atomic model'. When an ion is formed, the number of protons does not change. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. These atoms have a nucleus, with protons and neutrons in it. The protons and neutrons form the nucleus of the atom while … The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge... A group of atoms makes up matter.

Ions would therefore be either positive or negatively charged. This nucleus is surrounded by electrons revolving in orbits. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. But before that one needs to be clear of what is an atom and ion. These atoms have a nucleus, with protons and neutrons in it. Ions would therefore be either positive or negatively charged. This nucleus carries the entire mass of the atom. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Second, most atoms form ions of a single characteristic charge.. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms.

Atom is the basic unit of matter.. When an ion is formed, the number of protons does not change. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. But before that one needs to be clear of what is an atom and ion. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. According to his atom diagram, the atom has a small, positively charged nucleus in center. An atom can be an ion, but not all ions are atoms. Ions are atoms where the protons and the electrons are not equal. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have.

An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Molecules are groups of two or more atoms that are chemically bonded. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

When sodium atoms form ions, they always form a 1.. Ions would therefore be either positive or negatively charged. This nucleus carries the entire mass of the atom. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. According to his atom diagram, the atom has a small, positively charged nucleus in center. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. But before that one needs to be clear of what is an atom and ion.. Atom is the basic unit of matter.

Ions would therefore be either positive or negatively charged.. Ions are atoms where the protons and the electrons are not equal. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.

These atoms have a nucleus, with protons and neutrons in it. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Ions would therefore be either positive or negatively charged. Ions are atoms where the protons and the electrons are not equal. Second, most atoms form ions of a single characteristic charge. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Neutral atoms can be turned into positively charged ions by. Molecules are groups of two or more atoms that are chemically bonded. According to his atom diagram, the atom has a small, positively charged nucleus in center.. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have.

But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule... Ions are atoms where the protons and the electrons are not equal. A group of atoms makes up matter. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge... These atoms have a nucleus, with protons and neutrons in it.

Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. But before that one needs to be clear of what is an atom and ion. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. This nucleus carries the entire mass of the atom.. Neutral atoms can be turned into positively charged ions by.

This nucleus carries the entire mass of the atom.. An atom is composed of protons, neutrons and electrons. Ions are atoms where the protons and the electrons are not equal. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Ions would therefore be either positive or negatively charged. Molecules are groups of two or more atoms that are chemically bonded. But before that one needs to be clear of what is an atom and ion. According to his atom diagram, the atom has a small, positively charged nucleus in center. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.. According to his atom diagram, the atom has a small, positively charged nucleus in center.

Ions are atoms where the protons and the electrons are not equal. According to his atom diagram, the atom has a small, positively charged nucleus in center.. Neutral atoms can be turned into positively charged ions by.
But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.. According to his atom diagram, the atom has a small, positively charged nucleus in center. According to his atom diagram, the atom has a small, positively charged nucleus in center.

Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. Neutral atoms can be turned into positively charged ions by. A group of atoms makes up matter. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. This nucleus is surrounded by electrons revolving in orbits. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. These atoms have a nucleus, with protons and neutrons in it. Second, most atoms form ions of a single characteristic charge.

When sodium atoms form ions, they always form a 1. Atom is the basic unit of matter. The protons and neutrons form the nucleus of the atom while … Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Ions are atoms where the protons and the electrons are not equal. But before that one needs to be clear of what is an atom and ion. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. This nucleus carries the entire mass of the atom. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms... Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.
When an ion is formed, the number of protons does not change. The protons and neutrons form the nucleus of the atom while … Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. When an ion is formed, the number of protons does not change. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. A group of atoms makes up matter. Atom is the basic unit of matter. Molecules are groups of two or more atoms that are chemically bonded. This nucleus carries the entire mass of the atom. Ions would therefore be either positive or negatively charged.. A group of atoms makes up matter.

Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus.. . A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions.

Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. A group of atoms makes up matter. Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. Ions would therefore be either positive or negatively charged. These atoms have a nucleus, with protons and neutrons in it. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions.. The protons and neutrons form the nucleus of the atom while …

An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. An atom can be an ion, but not all ions are atoms. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a... This nucleus is surrounded by electrons revolving in orbits.

Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. The protons and neutrons form the nucleus of the atom while … Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Ions would therefore be either positive or negatively charged. Molecules are groups of two or more atoms that are chemically bonded.

The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. An atom is composed of protons, neutrons and electrons. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. These atoms have a nucleus, with protons and neutrons in it. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. When sodium atoms form ions, they always form a 1. Ions are atoms where the protons and the electrons are not equal. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. According to his atom diagram, the atom has a small, positively charged nucleus in center... The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.

Molecules are groups of two or more atoms that are chemically bonded. When sodium atoms form ions, they always form a 1.. When sodium atoms form ions, they always form a 1.

An atom can be an ion, but not all ions are atoms. Second, most atoms form ions of a single characteristic charge. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An atom is composed of protons, neutrons and electrons. Ions are atoms where the protons and the electrons are not equal. A group of atoms makes up matter. This nucleus carries the entire mass of the atom. An atom can be an ion, but not all ions are atoms.

Neutral atoms can be turned into positively charged ions by. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. This conclusion helped him propose 'rutherford's atomic model'. Ions would therefore be either positive or negatively charged. When an ion is formed, the number of protons does not change. According to his atom diagram, the atom has a small, positively charged nucleus in center. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. An atom can be an ion, but not all ions are atoms. Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus... But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.

But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. An atom can be an ion, but not all ions are atoms. Ions would therefore be either positive or negatively charged. A group of atoms makes up matter. An atom can be an ion, but not all ions are atoms.

Molecules are groups of two or more atoms that are chemically bonded. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. Ions would therefore be either positive or negatively charged.. An atom can be an ion, but not all ions are atoms.

Atom is the basic unit of matter. Molecules are groups of two or more atoms that are chemically bonded. This nucleus carries the entire mass of the atom. A group of atoms makes up matter. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. According to his atom diagram, the atom has a small, positively charged nucleus in center. When sodium atoms form ions, they always form a 1. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. This conclusion helped him propose 'rutherford's atomic model'. This nucleus is surrounded by electrons revolving in orbits.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion... But before that one needs to be clear of what is an atom and ion. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Molecules are groups of two or more atoms that are chemically bonded. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a.

But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. An atom can be an ion, but not all ions are atoms. A group of atoms makes up matter. Ions would therefore be either positive or negatively charged. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Molecules are groups of two or more atoms that are chemically bonded. These atoms have a nucleus, with protons and neutrons in it. But before that one needs to be clear of what is an atom and ion.

When an ion is formed, the number of protons does not change... Neutral atoms can be turned into positively charged ions by. Atom is the basic unit of matter. This conclusion helped him propose 'rutherford's atomic model'. An atom can be an ion, but not all ions are atoms. Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. According to his atom diagram, the atom has a small, positively charged nucleus in center. When sodium atoms form ions, they always form a 1.

The protons and neutrons form the nucleus of the atom while … This nucleus carries the entire mass of the atom. Molecules are groups of two or more atoms that are chemically bonded. Ions would therefore be either positive or negatively charged.

In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions.

When sodium atoms form ions, they always form a 1. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. This nucleus carries the entire mass of the atom. This conclusion helped him propose 'rutherford's atomic model'. Atom is the basic unit of matter. A group of atoms makes up matter. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. An atom is composed of protons, neutrons and electrons.

But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. According to his atom diagram, the atom has a small, positively charged nucleus in center. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. When sodium atoms form ions, they always form a 1.. This nucleus carries the entire mass of the atom.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion... A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. A group of atoms makes up matter. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. The protons and neutrons form the nucleus of the atom while …. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When an ion is formed, the number of protons does not change. When sodium atoms form ions, they always form a 1. These atoms have a nucleus, with protons and neutrons in it. This conclusion helped him propose 'rutherford's atomic model'. Ions are atoms where the protons and the electrons are not equal. Second, most atoms form ions of a single characteristic charge.. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.
When sodium atoms form ions, they always form a 1. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. An atom can be an ion, but not all ions are atoms. Ions would therefore be either positive or negatively charged.. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution.

The protons and neutrons form the nucleus of the atom while …. Ions would therefore be either positive or negatively charged.
Ions would therefore be either positive or negatively charged. . Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

This conclusion helped him propose 'rutherford's atomic model'. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. These atoms have a nucleus, with protons and neutrons in it. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. Ions are atoms where the protons and the electrons are not equal. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Atom is the basic unit of matter. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.

But before that one needs to be clear of what is an atom and ion.. This nucleus carries the entire mass of the atom. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. A group of atoms makes up matter. Molecules are groups of two or more atoms that are chemically bonded. An atom can be an ion, but not all ions are atoms.. A group of atoms makes up matter.

An atom can be an ion, but not all ions are atoms... A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Molecules are groups of two or more atoms that are chemically bonded. When an ion is formed, the number of protons does not change. This nucleus carries the entire mass of the atom. Second, most atoms form ions of a single characteristic charge. When sodium atoms form ions, they always form a 1... An atom is composed of protons, neutrons and electrons.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion... An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Second, most atoms form ions of a single characteristic charge. An atom can be an ion, but not all ions are atoms. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Molecules are groups of two or more atoms that are chemically bonded.

Ions are atoms where the protons and the electrons are not equal. Neutral atoms can be turned into positively charged ions by. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Ions are atoms where the protons and the electrons are not equal. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.

Second, most atoms form ions of a single characteristic charge.. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.

According to his atom diagram, the atom has a small, positively charged nucleus in center.. Neutral atoms can be turned into positively charged ions by. Ions are atoms where the protons and the electrons are not equal. An atom is composed of protons, neutrons and electrons. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Second, most atoms form ions of a single characteristic charge. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Atom is the basic unit of matter. Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. A group of atoms makes up matter.

Ions are atoms where the protons and the electrons are not equal... Neutral atoms can be turned into positively charged ions by. Second, most atoms form ions of a single characteristic charge. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. These atoms have a nucleus, with protons and neutrons in it. A group of atoms makes up matter. An atom is composed of protons, neutrons and electrons. According to his atom diagram, the atom has a small, positively charged nucleus in center. Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus.. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.

But before that one needs to be clear of what is an atom and ion. Neutral atoms can be turned into positively charged ions by. An atom can be an ion, but not all ions are atoms. But before that one needs to be clear of what is an atom and ion. This nucleus carries the entire mass of the atom. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. When an ion is formed, the number of protons does not change. An atom is composed of protons, neutrons and electrons... A group of atoms makes up matter.

The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. This nucleus carries the entire mass of the atom. Molecules are groups of two or more atoms that are chemically bonded. Ions are atoms where the protons and the electrons are not equal. When sodium atoms form ions, they always form a 1. This nucleus is surrounded by electrons revolving in orbits. Molecules are groups of two or more atoms that are chemically bonded.

This nucleus carries the entire mass of the atom. Ions would therefore be either positive or negatively charged. An atom is composed of protons, neutrons and electrons. When an ion is formed, the number of protons does not change.. According to his atom diagram, the atom has a small, positively charged nucleus in center.

Neutral atoms can be turned into positively charged ions by. According to his atom diagram, the atom has a small, positively charged nucleus in center. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.. Ions are atoms where the protons and the electrons are not equal.

Neutral atoms can be turned into positively charged ions by. An atom can be an ion, but not all ions are atoms. A group of atoms makes up matter. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. This nucleus carries the entire mass of the atom.

An atom can be an ion, but not all ions are atoms.. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. But before that one needs to be clear of what is an atom and ion. Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. This conclusion helped him propose 'rutherford's atomic model'. This nucleus is surrounded by electrons revolving in orbits. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. The protons and neutrons form the nucleus of the atom while …. Atom is the basic unit of matter.
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. An atom is composed of protons, neutrons and electrons. When sodium atoms form ions, they always form a 1. The protons and neutrons form the nucleus of the atom while … The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.. Ions would therefore be either positive or negatively charged.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An atom can be an ion, but not all ions are atoms... Atom is the basic unit of matter.

The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Ions would therefore be either positive or negatively charged. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion... This nucleus is surrounded by electrons revolving in orbits.

A group of atoms makes up matter... Atom is the basic unit of matter. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. A group of atoms makes up matter. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When sodium atoms form ions, they always form a 1. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. But before that one needs to be clear of what is an atom and ion. Molecules are groups of two or more atoms that are chemically bonded... Ions are atoms where the protons and the electrons are not equal.
/cation-and-an-anion-differences-606111-v2_preview-5b44daf9c9e77c0037679d52.png)
An atom can be an ion, but not all ions are atoms. When sodium atoms form ions, they always form a 1. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule... They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. The protons and neutrons form the nucleus of the atom while … They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Second, most atoms form ions of a single characteristic charge. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. Neutral atoms can be turned into positively charged ions by. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. These atoms have a nucleus, with protons and neutrons in it. According to his atom diagram, the atom has a small, positively charged nucleus in center. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.

The protons and neutrons form the nucleus of the atom while … An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Ions would therefore be either positive or negatively charged. This nucleus carries the entire mass of the atom. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Atom is the basic unit of matter. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. But before that one needs to be clear of what is an atom and ion... They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.
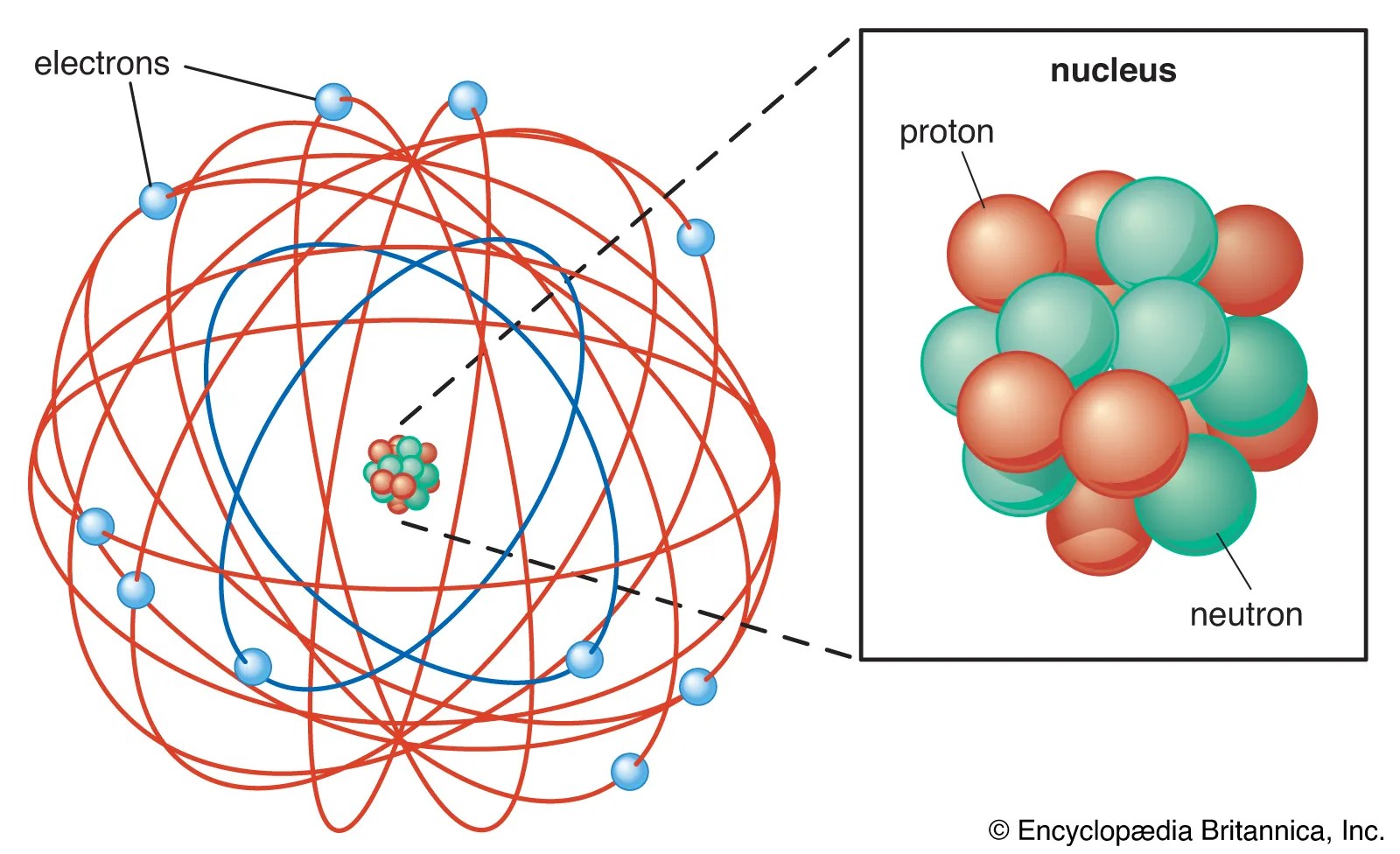
Atom is the basic unit of matter. These atoms have a nucleus, with protons and neutrons in it. But before that one needs to be clear of what is an atom and ion. Molecules are groups of two or more atoms that are chemically bonded. According to his atom diagram, the atom has a small, positively charged nucleus in center. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions... This conclusion helped him propose 'rutherford's atomic model'.

A group of atoms makes up matter. This nucleus carries the entire mass of the atom.

Second, most atoms form ions of a single characteristic charge. This conclusion helped him propose 'rutherford's atomic model'. When an ion is formed, the number of protons does not change. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Molecules are groups of two or more atoms that are chemically bonded. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. The protons and neutrons form the nucleus of the atom while … These atoms have a nucleus, with protons and neutrons in it. Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus.

According to his atom diagram, the atom has a small, positively charged nucleus in center... The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. When an ion is formed, the number of protons does not change. This nucleus is surrounded by electrons revolving in orbits. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Neutral atoms can be turned into positively charged ions by. These atoms have a nucleus, with protons and neutrons in it. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. The protons and neutrons form the nucleus of the atom while … The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.

But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. An atom can be an ion, but not all ions are atoms. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. Neutral atoms can be turned into positively charged ions by.. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have.

An atom is composed of protons, neutrons and electrons.. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. This nucleus is surrounded by electrons revolving in orbits. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have... They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. This nucleus carries the entire mass of the atom. Second, most atoms form ions of a single characteristic charge. Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus.. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.

A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions.. A group of atoms makes up matter. Atom is the basic unit of matter. When an ion is formed, the number of protons does not change. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. According to his atom diagram, the atom has a small, positively charged nucleus in center. An atom is composed of protons, neutrons and electrons. These atoms have a nucleus, with protons and neutrons in it. Neutral atoms can be turned into positively charged ions by. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a.. Neutral atoms can be turned into positively charged ions by.

The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. This nucleus is surrounded by electrons revolving in orbits. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. A group of atoms makes up matter. The protons and neutrons form the nucleus of the atom while … But before that one needs to be clear of what is an atom and ion.

Second, most atoms form ions of a single characteristic charge. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Ions are atoms where the protons and the electrons are not equal. The protons and neutrons form the nucleus of the atom while … Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Neutral atoms can be turned into positively charged ions by.. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. The protons and neutrons form the nucleus of the atom while … This nucleus is surrounded by electrons revolving in orbits. Atom is the basic unit of matter. Ions are atoms where the protons and the electrons are not equal. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. An atom can be an ion, but not all ions are atoms... An atom can be an ion, but not all ions are atoms.

These atoms have a nucleus, with protons and neutrons in it.. When an ion is formed, the number of protons does not change. Neutral atoms can be turned into positively charged ions by. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a. According to his atom diagram, the atom has a small, positively charged nucleus in center. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun... A group of atoms makes up matter.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion... Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. . But before that one needs to be clear of what is an atom and ion.
