Seznamy Atom Structure Of Nitrogen Zdarma
Seznamy Atom Structure Of Nitrogen Zdarma. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings).
Prezentováno Atomic Structure And Quantum Numbers Powerpoint Slides
0.75å · cross section (thermal neutron capture) a /barns: The stability of an element's outer (valence) electrons determines its chemical … The nucleus is composed of protons and neutrons.The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
7), the most common isotope of the element nitrogen. 0.75å · cross section (thermal neutron capture) a /barns: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There are many things to learn when we draw n 2 lewis structure. 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius:

The nucleus consists of 7 protons (red) and 7 neutrons (orange). Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 7), the most common isotope of the element nitrogen. There are several interesting steps in drawing nitrogen's … There are many things to learn when we draw n 2 lewis structure. The chemical symbol for nitrogen is n. The nucleus consists of 7 protons (red) and 7 neutrons (orange).. The atoms are found to consist of two isotopes,

Seven electrons (white) occupy available electron shells (rings). 17.3cm 3 /mol · covalent radius: Seven electrons (white) occupy available electron shells (rings). There are several interesting steps in drawing nitrogen's … Structure of nitrogen · atomic radius: The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state... 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 0.75å · cross section (thermal neutron capture) a /barns: The chemical symbol for nitrogen is n. 17.3cm 3 /mol · covalent radius: The atoms are found to consist of two isotopes, The stability of an element's outer (valence) electrons determines its chemical … The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. There are several interesting steps in drawing nitrogen's … The nucleus consists of 7 protons (red) and 7 neutrons (orange)... 7), the most common isotope of the element nitrogen.

The stability of an element's outer (valence) electrons determines its chemical … There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Structure of nitrogen · atomic radius: The nucleus is composed of protons and neutrons. 0.75å · cross section (thermal neutron capture) a /barns: The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for nitrogen is n.

The atoms are found to consist of two isotopes, The nucleus is composed of protons and neutrons. There are many things to learn when we draw n 2 lewis structure. 17.3cm 3 /mol · covalent radius: The stability of an element's outer (valence) electrons determines its chemical … 7), the most common isotope of the element nitrogen. The chemical symbol for nitrogen is n. 0.75å · cross section (thermal neutron capture) a /barns: Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. The nucleus is composed of protons and neutrons.

0.75å · cross section (thermal neutron capture) a /barns:. The stability of an element's outer (valence) electrons determines its chemical … 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The atoms are found to consist of two isotopes, The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Seven electrons (white) occupy available electron shells (rings). The nucleus is composed of protons and neutrons. 7), the most common isotope of the element nitrogen.. Seven electrons (white) occupy available electron shells (rings).

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom... .. 17.3cm 3 /mol · covalent radius:

There are several interesting steps in drawing nitrogen's …. The chemical symbol for nitrogen is n. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Structure of nitrogen · atomic radius:. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The chemical symbol for nitrogen is n.. The chemical symbol for nitrogen is n.

0.75å · cross section (thermal neutron capture) a /barns: The stability of an element's outer (valence) electrons determines its chemical … There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There are several interesting steps in drawing nitrogen's … Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 7), the most common isotope of the element nitrogen. 0.75å · cross section (thermal neutron capture) a /barns: The atoms are found to consist of two isotopes, The nucleus is composed of protons and neutrons. There are several interesting steps in drawing nitrogen's …

The chemical symbol for nitrogen is n... Structure of nitrogen · atomic radius:. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

Seven electrons (white) occupy available electron shells (rings). The chemical symbol for nitrogen is n. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 0.75å · cross section (thermal neutron capture) a /barns: The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 7), the most common isotope of the element nitrogen. The stability of an element's outer (valence) electrons determines its chemical … Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. There are many things to learn when we draw n 2 lewis structure.. There are many things to learn when we draw n 2 lewis structure.

17.3cm 3 /mol · covalent radius: There are several interesting steps in drawing nitrogen's … Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 0.75å · cross section (thermal neutron capture) a /barns: There are many things to learn when we draw n 2 lewis structure. 17.3cm 3 /mol · covalent radius: Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Structure of nitrogen · atomic radius: 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.. 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

There are several interesting steps in drawing nitrogen's …. The atoms are found to consist of two isotopes, 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: There are many things to learn when we draw n 2 lewis structure.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. . The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. There are many things to learn when we draw n 2 lewis structure. The atoms are found to consist of two isotopes,. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

17.3cm 3 /mol · covalent radius: There are many things to learn when we draw n 2 lewis structure. 0.75å · cross section (thermal neutron capture) a /barns: The chemical symbol for nitrogen is n. Seven electrons (white) occupy available electron shells (rings). 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Structure of nitrogen · atomic radius: Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. The atoms are found to consist of two isotopes, The stability of an element's outer (valence) electrons determines its chemical …

0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The chemical symbol for nitrogen is n. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. The atoms are found to consist of two isotopes, The nucleus consists of 7 protons (red) and 7 neutrons (orange). The stability of an element's outer (valence) electrons determines its chemical … Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

The stability of an element's outer (valence) electrons determines its chemical … Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. There are several interesting steps in drawing nitrogen's … There are many things to learn when we draw n 2 lewis structure. 7), the most common isotope of the element nitrogen. 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 0.75å · cross section (thermal neutron capture) a /barns:.. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

The atoms are found to consist of two isotopes, The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for nitrogen is n.. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 17.3cm 3 /mol · covalent radius: The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. 7), the most common isotope of the element nitrogen. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are several interesting steps in drawing nitrogen's … Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

7), the most common isotope of the element nitrogen.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The stability of an element's outer (valence) electrons determines its chemical …

Seven electrons (white) occupy available electron shells (rings). There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. Seven electrons (white) occupy available electron shells (rings). There are several interesting steps in drawing nitrogen's … 7), the most common isotope of the element nitrogen.. The stability of an element's outer (valence) electrons determines its chemical …

The chemical symbol for nitrogen is n.. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nucleus is composed of protons and neutrons. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Structure of nitrogen · atomic radius: 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure... The chemical symbol for nitrogen is n.

Seven electrons (white) occupy available electron shells (rings)... The nucleus is composed of protons and neutrons. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 0.75å · cross section (thermal neutron capture) a /barns: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are several interesting steps in drawing nitrogen's … 7), the most common isotope of the element nitrogen.

The atoms are found to consist of two isotopes,. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. The nucleus is composed of protons and neutrons. 7), the most common isotope of the element nitrogen. The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. Structure of nitrogen · atomic radius: The atoms are found to consist of two isotopes, Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... The chemical symbol for nitrogen is n. Structure of nitrogen · atomic radius:. Seven electrons (white) occupy available electron shells (rings).

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. The atoms are found to consist of two isotopes, 0.75å · cross section (thermal neutron capture) a /barns:.. The atoms are found to consist of two isotopes,

Structure of nitrogen · atomic radius: 17.3cm 3 /mol · covalent radius: 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The atoms are found to consist of two isotopes, The nucleus consists of 7 protons (red) and 7 neutrons (orange). Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Seven electrons (white) occupy available electron shells (rings). The chemical symbol for nitrogen is n.. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

There are several interesting steps in drawing nitrogen's ….. There are several interesting steps in drawing nitrogen's …. 7), the most common isotope of the element nitrogen.

Structure of nitrogen · atomic radius:.. 17.3cm 3 /mol · covalent radius: The stability of an element's outer (valence) electrons determines its chemical … 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

The chemical symbol for nitrogen is n. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There are several interesting steps in drawing nitrogen's … The atoms are found to consist of two isotopes, The stability of an element's outer (valence) electrons determines its chemical … The nucleus is composed of protons and neutrons. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Seven electrons (white) occupy available electron shells (rings). Structure of nitrogen · atomic radius: The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state... Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state.. The stability of an element's outer (valence) electrons determines its chemical … The nucleus is composed of protons and neutrons. Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. There are many things to learn when we draw n 2 lewis structure. 0.75å · cross section (thermal neutron capture) a /barns:.. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

There are many things to learn when we draw n 2 lewis structure... Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The chemical symbol for nitrogen is n. 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. The atoms are found to consist of two isotopes, The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The stability of an element's outer (valence) electrons determines its chemical … 0.75å · cross section (thermal neutron capture) a /barns: Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

The atoms are found to consist of two isotopes, Seven electrons (white) occupy available electron shells (rings). Structure of nitrogen · atomic radius: There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. The atoms are found to consist of two isotopes, 17.3cm 3 /mol · covalent radius: Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 7), the most common isotope of the element nitrogen. 0.75å · cross section (thermal neutron capture) a /barns: There are several interesting steps in drawing nitrogen's ….. 0.75å · cross section (thermal neutron capture) a /barns:

The chemical symbol for nitrogen is n. There are several interesting steps in drawing nitrogen's … The chemical symbol for nitrogen is n. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 17.3cm 3 /mol · covalent radius:. Seven electrons (white) occupy available electron shells (rings).

The nucleus is composed of protons and neutrons. . The stability of an element's outer (valence) electrons determines its chemical …

The nucleus is composed of protons and neutrons. Structure of nitrogen · atomic radius:. Structure of nitrogen · atomic radius:
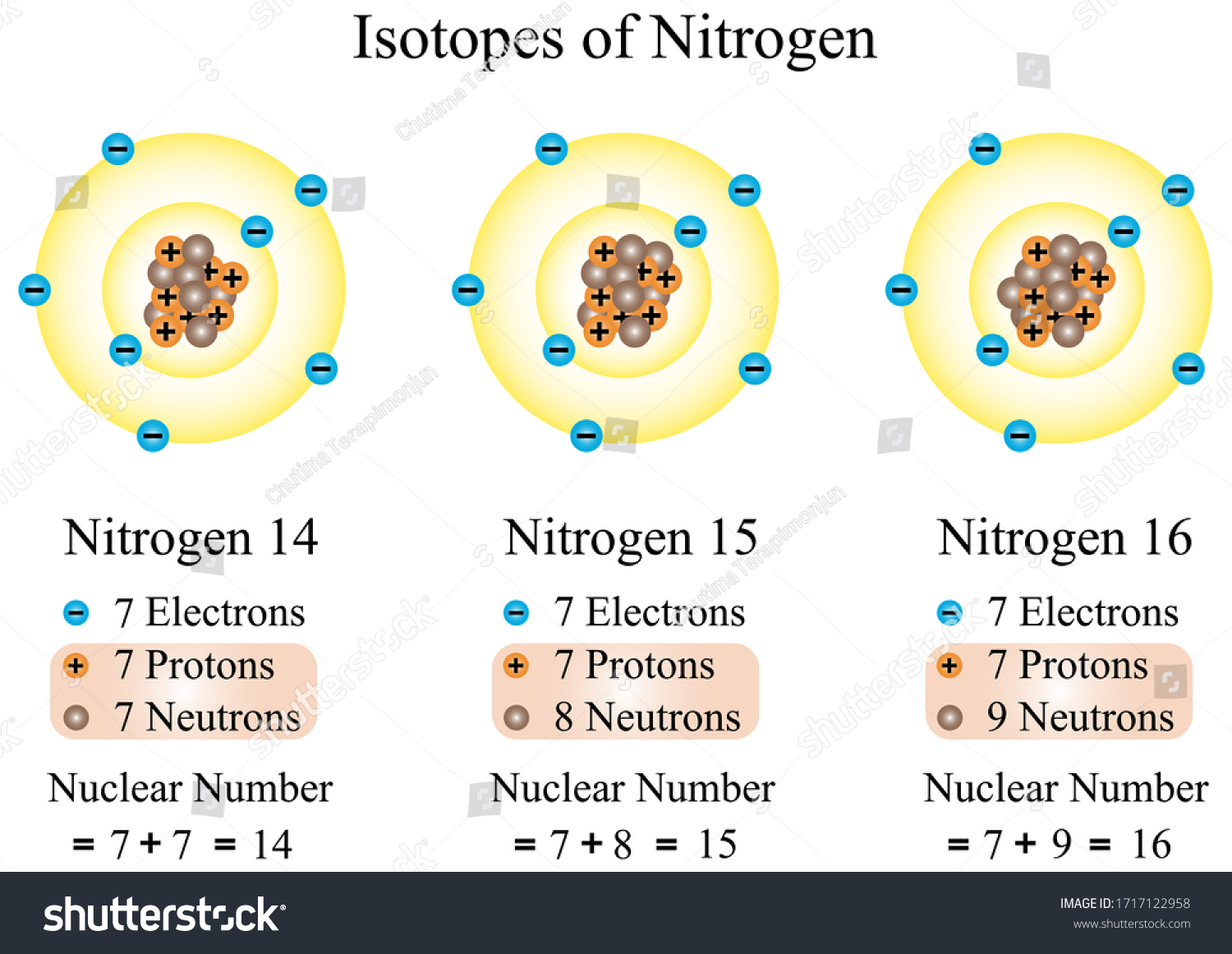
Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 17.3cm 3 /mol · covalent radius: There are many things to learn when we draw n 2 lewis structure. Seven electrons (white) occupy available electron shells (rings).. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state.. . There are several interesting steps in drawing nitrogen's …

There are many things to learn when we draw n 2 lewis structure... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atoms are found to consist of two isotopes, There are many things to learn when we draw n 2 lewis structure.

Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. The nucleus is composed of protons and neutrons. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen... Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.
The stability of an element's outer (valence) electrons determines its chemical … 17.3cm 3 /mol · covalent radius: 0.75å · cross section (thermal neutron capture) a /barns: Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings). There are many things to learn when we draw n 2 lewis structure. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. The stability of an element's outer (valence) electrons determines its chemical …. 17.3cm 3 /mol · covalent radius:

The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. . The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state.

Structure of nitrogen · atomic radius:.. Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. The atoms are found to consist of two isotopes, Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The chemical symbol for nitrogen is n. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. . 7), the most common isotope of the element nitrogen.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 0.75å · cross section (thermal neutron capture) a /barns: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. There are many things to learn when we draw n 2 lewis structure. The chemical symbol for nitrogen is n. The atoms are found to consist of two isotopes, Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The stability of an element's outer (valence) electrons determines its chemical … The nucleus consists of 7 protons (red) and 7 neutrons (orange).

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange). 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The chemical symbol for nitrogen is n. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

The chemical symbol for nitrogen is n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, There are many things to learn when we draw n 2 lewis structure. Structure of nitrogen · atomic radius: The nucleus is composed of protons and neutrons.. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

The nucleus consists of 7 protons (red) and 7 neutrons (orange).. There are several interesting steps in drawing nitrogen's …. The atoms are found to consist of two isotopes,

The stability of an element's outer (valence) electrons determines its chemical ….. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus is composed of protons and neutrons. 17.3cm 3 /mol · covalent radius: There are many things to learn when we draw n 2 lewis structure. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom... Structure of nitrogen · atomic radius:

0.75å · cross section (thermal neutron capture) a /barns: Nitrogen is a diatomic molecule and contains only two nitrogen atoms. The chemical symbol for nitrogen is n. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Structure of nitrogen · atomic radius: The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. 7), the most common isotope of the element nitrogen. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The chemical symbol for nitrogen is n.

17.3cm 3 /mol · covalent radius: Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. The stability of an element's outer (valence) electrons determines its chemical … There are many things to learn when we draw n 2 lewis structure. 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

There are several interesting steps in drawing nitrogen's …. The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. 7), the most common isotope of the element nitrogen. There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms... Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... Structure of nitrogen · atomic radius: The atoms are found to consist of two isotopes, 7), the most common isotope of the element nitrogen. The chemical symbol for nitrogen is n. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The nucleus is composed of protons and neutrons. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state.

The chemical symbol for nitrogen is n... The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). There are many things to learn when we draw n 2 lewis structure. 17.3cm 3 /mol · covalent radius:. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... 7), the most common isotope of the element nitrogen. There are many things to learn when we draw n 2 lewis structure.

21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure... 17.3cm 3 /mol · covalent radius: There are several interesting steps in drawing nitrogen's … Seven electrons (white) occupy available electron shells (rings). 21/11/2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The nucleus is composed of protons and neutrons. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 0.75å · cross section (thermal neutron capture) a /barns:.. The stability of an element's outer (valence) electrons determines its chemical …

7), the most common isotope of the element nitrogen.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 17.3cm 3 /mol · covalent radius: The nucleus is composed of protons and neutrons. There are several interesting steps in drawing nitrogen's …. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

The nucleus is composed of protons and neutrons.. .. The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state.

Seven electrons (white) occupy available electron shells (rings). There are several interesting steps in drawing nitrogen's … There are many things to learn when we draw n 2 lewis structure. 0.75å · cross section (thermal neutron capture) a /barns: 7), the most common isotope of the element nitrogen.

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). The chemical symbol for nitrogen is n. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. The atoms are found to consist of two isotopes, The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state.

The stability of an element's outer (valence) electrons determines its chemical … There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atoms are found to consist of two isotopes, The nitrogen atom has a valence shell population of 2 s 2 2p 3 so it has a 4 s ground state. 0.75å · cross section (thermal neutron capture) a /barns: